3 Brands Of High Blood Pressure Drugs Recalled On 29 Mar
On Thursday (28 Mar), the Health Sciences Authority (HSA) recalled 3 brands of high blood pressure medicine containing “losartan potassium”.
3 High Blood Pressure Drugs May Pose Cancer Risk; HSA Recall Affects 137,000 S’pore Patients
These drugs were found with “higher than acceptable” levels of an impurity which may increase the risk of developing cancer.
The recall is expected to affect 137,000 patients in Singapore who were prescribed the affected drugs.
One of the affected patients, Mr Zhou, took to Facebook shortly after news broke on Friday (29 Mar).
Having consumed the drugs for the past 2-3 years, he was noticeably upset.
Here’s his post in full.
English translation:
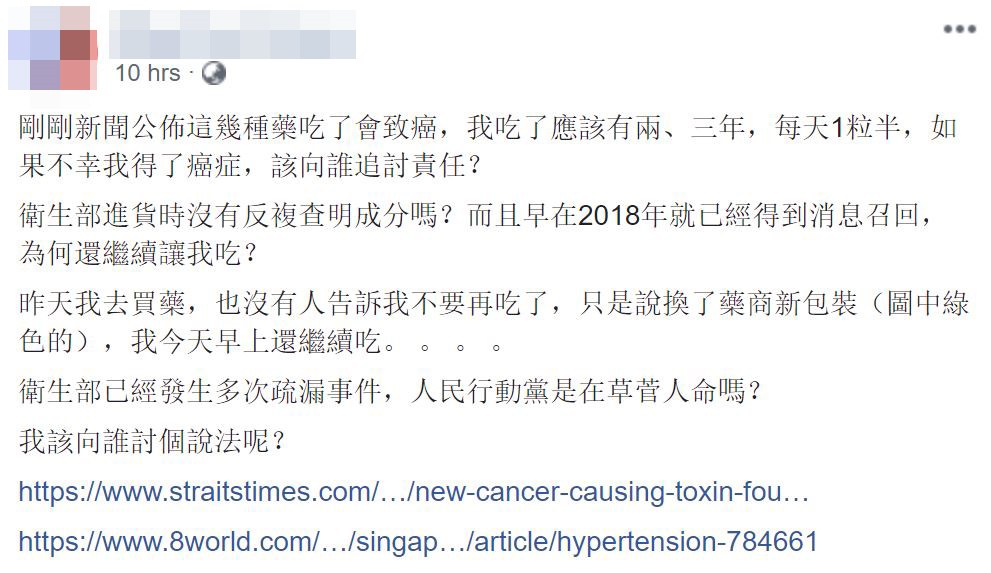
The HSA just announced that these medications may lead to cancer. I have been consuming them for around 2-3 years, having 1.5 pills daily.
In the unfortunate event where I develop cancer, who is going to be responsible?
Did the HSA not conduct checks on the pills’ content when they are importing them?
Additionally, the same medications were recalled once in 2018. Why did the HSA still allow me to consume the pills?
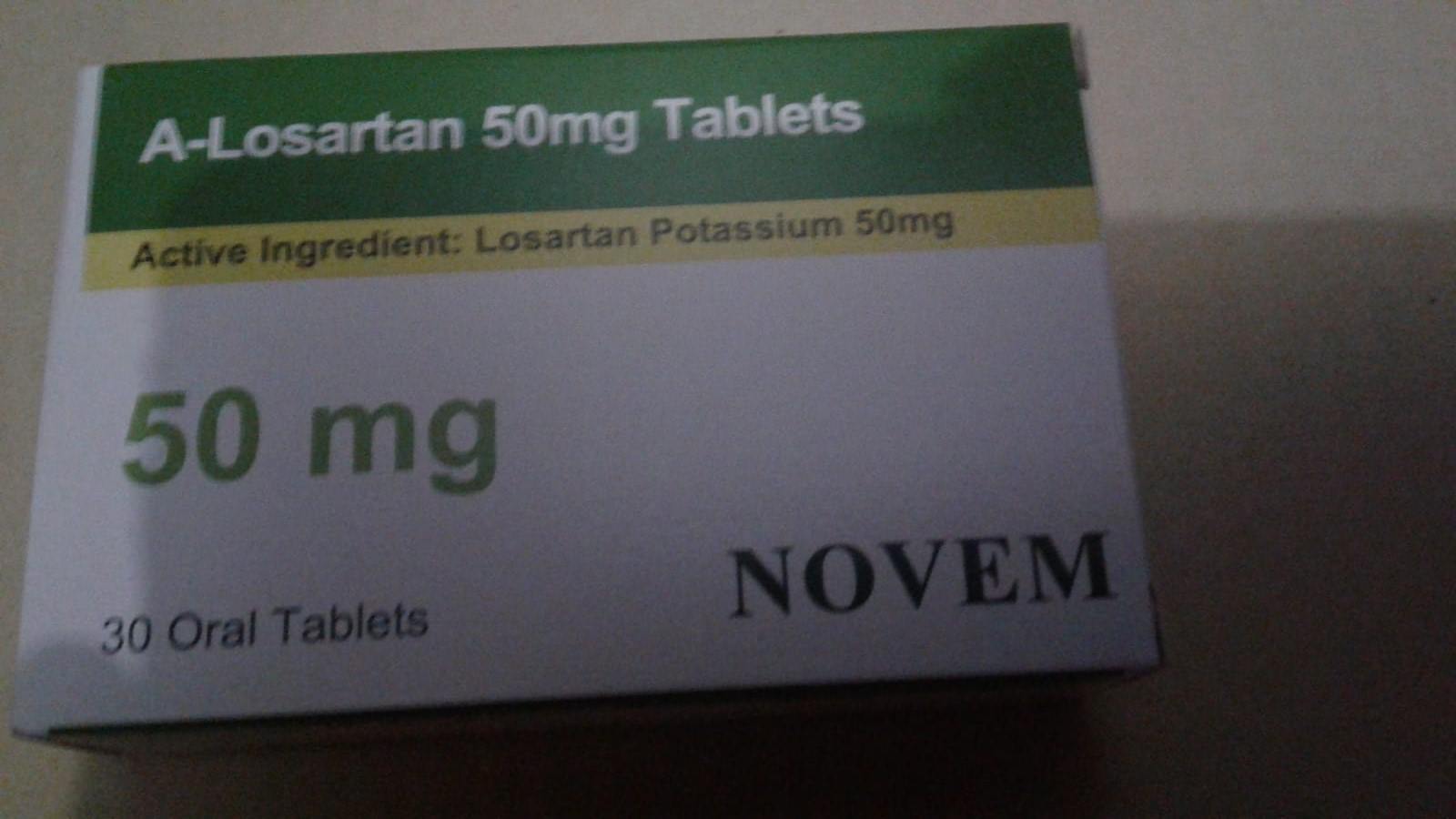
Yesterday when I purchased the medicine, I was informed of the medicine’s new packaging, but no one told me to stop consuming. I was still eating the medicine this morning.
This isn’t the first time that such lapses have happened to the HSA. Is the ruling party toying with our lives?
Who should I turn to for an explanation?
Consumed pills daily for 2-3 years
Mr Zhou claims that he has consumed the recalled drugs for the past 2-3 years, during which he would consume 1.5 pills daily.
As such, he is concerned that he’s at a higher risk of having cancer, as a result of taking these pills.
Coincidentally, Mr Zhou purchased one of the affected drugs 1 day before the announcement and received notice about the drug’s new packaging, but was not told to stop consuming them.
He claimed to have consumed the drugs in the morning of the day when the announcement was made.
Mr Zhou also added that the same drug was recalled in 2018. Thus, the authorities should have stopped patients from consuming the drugs at an earlier date.
He was most likely making reference to a recall last year, where the US drug administration recalled losartan tablets due to another cancer-causing impurity.
The impurity that raised concerns then – NDEA – is different from the substance responsible for the recall in Singapore — nitrosamine.
Slight risk of cancer
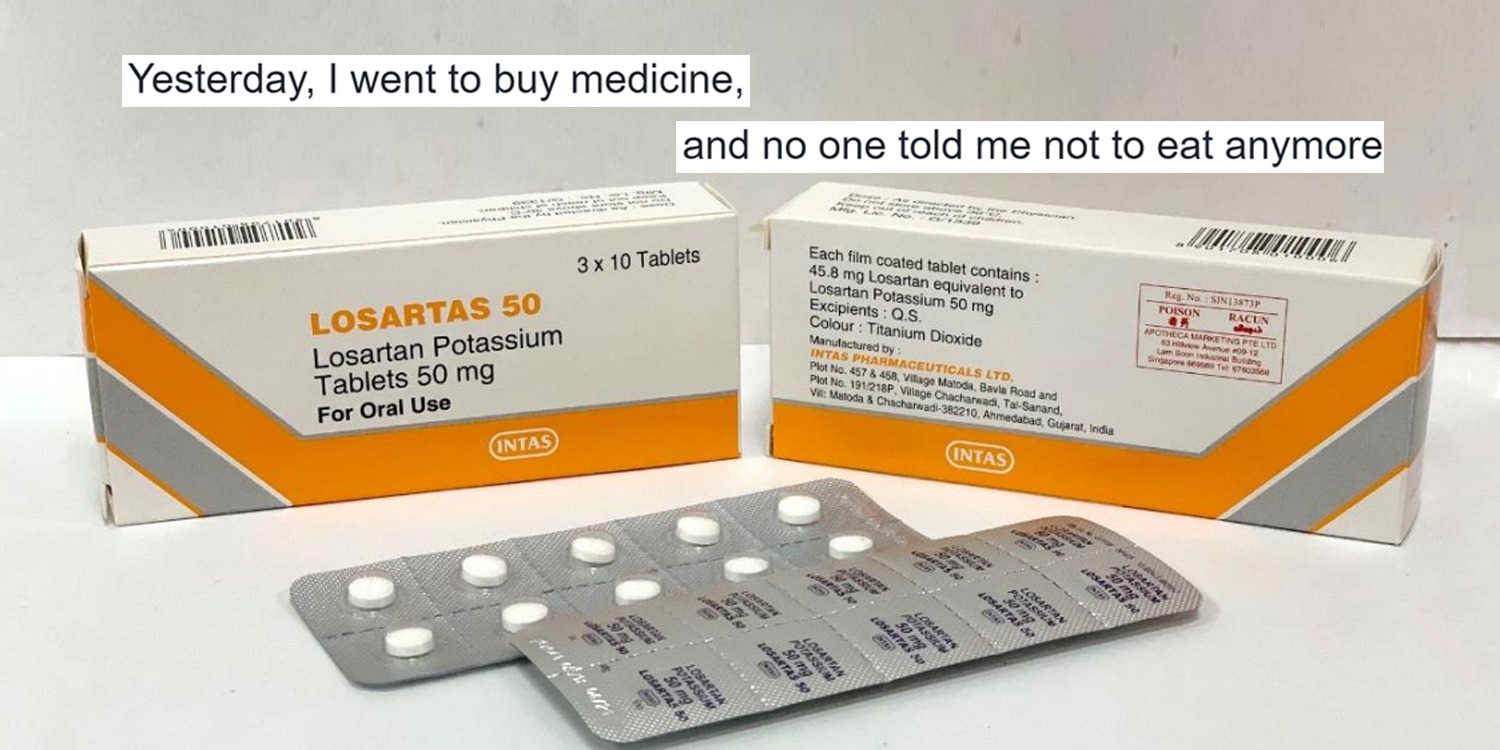
The 3 brands of recalled drugs are as follows:
- Losartas – 50mg, 100mg
- Losagen – 50mg, 100mg
- Hyperten – 50mg, 100mg
Losartas tablets are available at public and private healthcare institutes while the remaining two are dispensed at private clinics.
HSA made the urgent recall after “trace amounts of nitrosamine impurities” were found in these products.
Long-term exposure to these impurities may lead to a higher risk of developing cancer.
That said, the risk of developing cancer after being exposed to nitrosamine is estimated to be less than 0.0002%, after a 6-month exposure.
HSA warned that patients who were prescribed the affected drugs should continue with consuming medicine, as stopping treatment could post “greater immediate risk” to their health.
Do continue taking your medicine
Given the sheer number of people affected by the recall, perhaps it would have been better for news of the recall to be more gently disseminated, to avoid panic.
That said, those who were previously prescribed the affected drugs are strongly advised to continue with their treatment plans.
Featured image from Health Sciences Authority and Facebook.