137,000 High Blood Pressure Patients In S’pore Should Still Take Their Medicine, Says HSA
The Health Sciences Authority (HSA) has announced the urgent recall of 3 kinds of high blood pressure medicine containing “losartan potassium” on Friday (29 Mar).
Authorities found “trace amounts of nitrosamine impurities” in these products and that long-term exposure may “potentially increase the risk of cancer”.
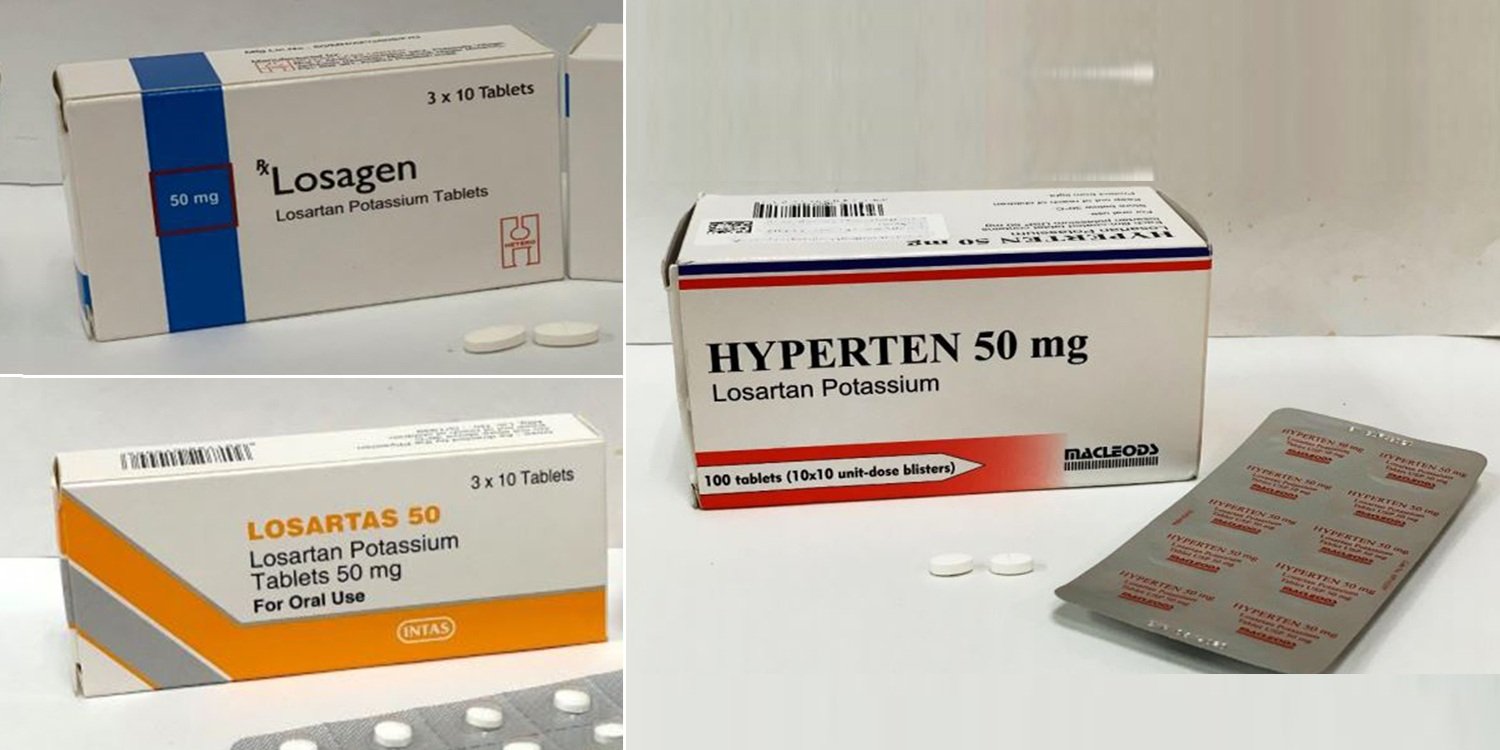
These are the 3 brands of recalled drugs & the healthcare institutes they’re available at:
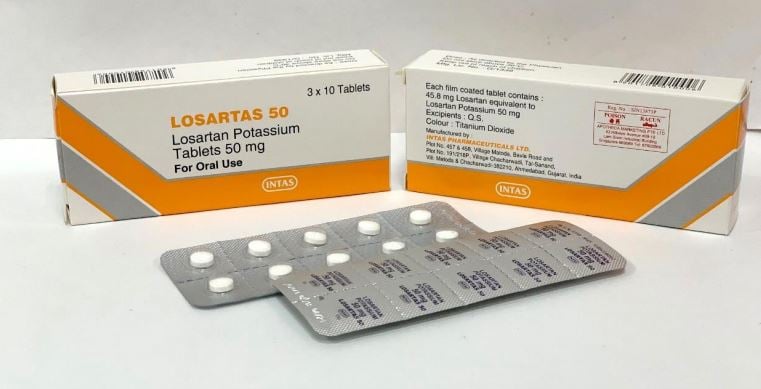
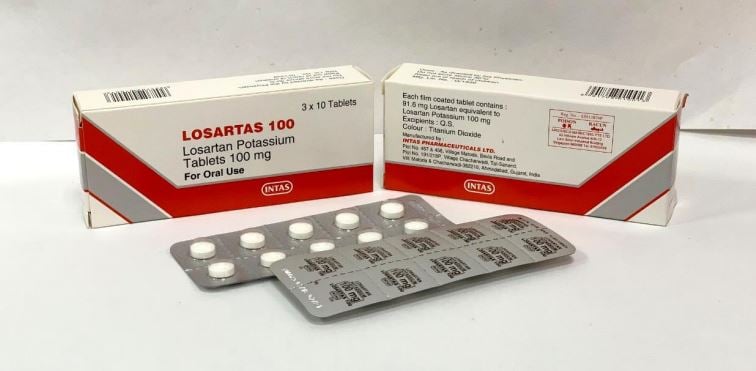
- Losartas – 50mg, 100mg (Public & Private)
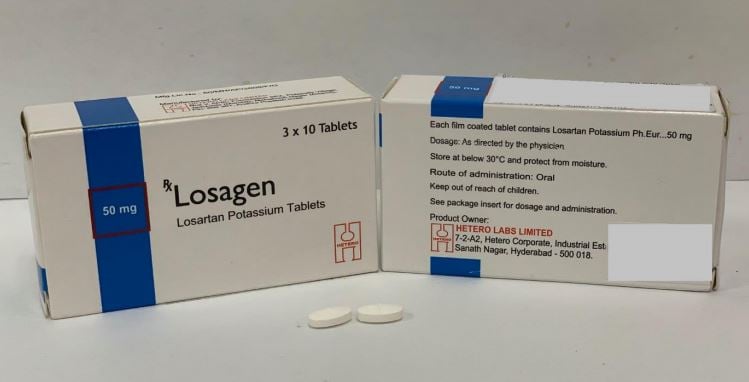
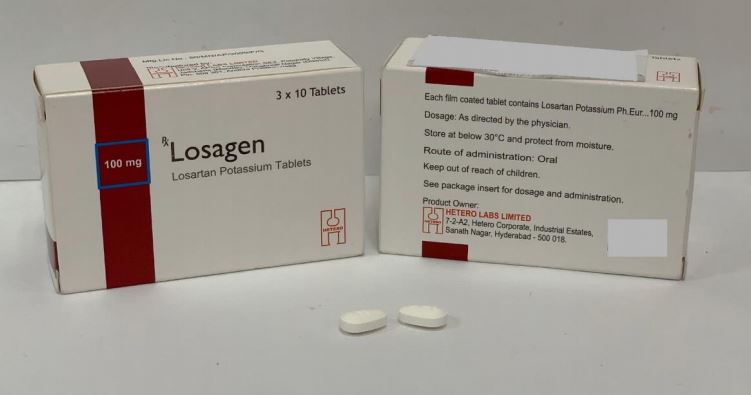
- Losagen – 50mg, 100mg (Private)
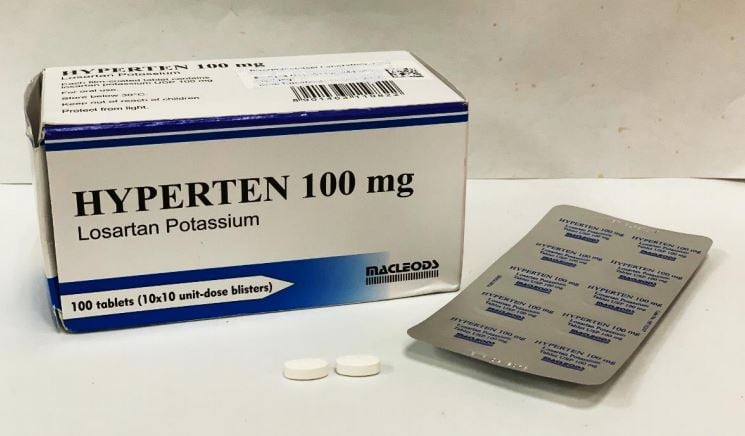
- Hyperten – 50mg, 100mg (Private)
Do note that affected patients shouldn’t stop taking their medicine as HSA warns that ceasing treatment could post “greater immediate risk” to your health.
Recall impacts 137,000 patients
The Ministry of Health (MOH) estimates that close to 137,000 people are currently using the affected 3 brands of drugs.
You can recognise the tablets affected by their packaging as pictured below.
Losartas – 50mg, 100mg
Losagen – 50mg, 100mg
Hyperten – 50mg, 100mg
Active ingredient “losartan potassium” which induced the recall was traced back to a company named Hetero Labs originating from India.
Only less than 0.0002% cancer risk
There’s no need to panic, however.
High exposure to nitrosamines over a long period of time, may increase your risk of cancer. But even after 6 months, the estimated risk is less than 0.0002%.
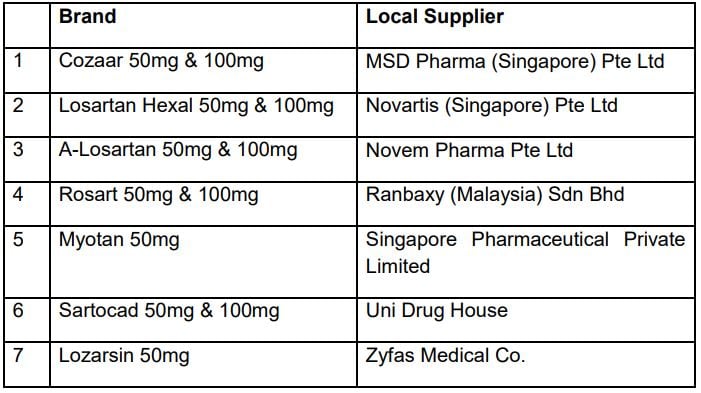
7 brands are clear
Here are 7 brands of losartan drugs sold in Singapore that have been given the all-clear:
Hypertension is common in Singapore
About 1 in 4 Singaporeans aged 30-69 have hypertension, according to the Ministry of Health.
High blood pressure is also more common in elderly folk.
There’s a 50% chance you’ll be experiencing this condition once you hit the ’60 to 69-year-old’ age range in Singapore.
Keep taking your meds regularly
If you know anyone affected by the recall, or are concerned if your medicine contains this ingredient, do reach out to your doctors to review your treatment plans.
For patients who are due to visit public health institutes before 1 Jul, MOH confirms that your doctors will prescribe “alternative medicines” accordingly.
Patients with appointments after 1 Jul should wait for public hospital or polyclinic staff to contact you regarding an earlier consult.
Everyone’s reminded to keep taking your medicine regularly, especially if you’re a chronic high blood pressure patient.
Do share this with your friends and family so they will know exactly what steps to take regarding the recall.
Featured image from Health Sciences Authority.

Drop us your email so you won't miss the latest news.